BP Gulf Oil Spill Disaster, An Impossible Well to Cap?
Politics / Environmental Issues Jul 03, 2010 - 05:40 AM GMTBy: Joshua_S_Burnett

 My first article in this series was a query; I listed the theories and evidence I’d seen thus far and asked for readers to respond and tell me I was crazy (which a few of you did, in as many words…much appreciated J). I felt that what I was writing about seemed to me far “too apocalyptic” as one comment on the original article stated. If you read the introduction and conclusion of the first article it becomes rather obvious that I wasn’t advocating, I was inquiring. I received over 100 emails; after investigating those leads here are what I’ve concluded thus far.
My first article in this series was a query; I listed the theories and evidence I’d seen thus far and asked for readers to respond and tell me I was crazy (which a few of you did, in as many words…much appreciated J). I felt that what I was writing about seemed to me far “too apocalyptic” as one comment on the original article stated. If you read the introduction and conclusion of the first article it becomes rather obvious that I wasn’t advocating, I was inquiring. I received over 100 emails; after investigating those leads here are what I’ve concluded thus far.
There are three main sources of threats: oil, gas, and dispersants. Let’s examine each in turn.
Oil
The most asked question that I received concerned the statement in my previous article about oil raining down. Many folks were quick to accurately point out that oil is not transmitted through evaporation into a normal rain cycle (some oil is evaporated, but the amount is negligible). The concern I have is not evaporation but rather the extreme winds that occur in the midst of hurricanes and tropical storms; the lowest wind possible in the hurricane category is 74 mph; this is sufficient to pick up and transmit living fish, frogs, squid, etc miles inland (and such has happened multiple times throughout history); if objects weighing pounds can be transported this way it is no stretch of the imagination to see that molecules weighing milligrams can experience the same phenomenon and to a much further distance.
Another common argument was that water in hurricanes is evaporated and therefore pure; if it weren’t then the southernmost parts of Gulf States would be salt-poisoned and inhospitable to flora and fauna. This argument fails on a couple of key points: the first is that while condensation from hurricanes is primarily evaporated it is not exclusively so. Winds for hurricanes can exceed 155 mph; these storm systems commonly spawn tornados (over land) and water spouts (over water). A not insignificant portion of salt water is swept up into the storm, but the reason why this doesn’t have a greater effect on the land is due to the dilution rate. While the average salinity of ocean water is 3.5%, the salinity of human blood is 0.9% meaning that we would only have to dilute the salt water 4:1 to make an isotonic solution safe to drink. Even if we went further and decreased the salt content to what the accepted standard is for city drinking water (0.1%) we would only have to dilute it 34:1. Even if as much as 3% of the rain coming from a hurricane was salt water that was swept up and not evaporated it would still approximate the purity standards of drinking water. The rain could approximate a 10:1 solution and still be safe for humans to drink. Conversely 1 quart of oil can contaminate 250,000 gallons of water, for a dilution rate of 1,000,000:1 which is still toxic.
This may sound artificially high though; most of us don’t have a great reference point for large amounts of water. I had several folks berate me because they didn’t see how this could be true because the water supplies of many municipalities are fed by bodies of water open to public fishing and therefore frequented by motored watercraft.; they assumed that this water would be as contaminated by spilled oil as any. The first answer I gave them was that this water is filtered before being consumed by anyone in the municipality; an equally appropriate answer would again refer to dilution. An Olympic size swimming pool will hold approximately 600,000 gallons of water, meaning that someone could dump a half gallon of crude into the pool and it still wouldn’t be considered toxic. Of course judging by how fast the pool clears when a little kid blows chunks in the shallow end most people probably aren’t comfortable even with those standards of cleanliness J. How much oil will rain down on land is impossible to predict due to the size of surface spills, effects of dispersants, size of the storm, strength of the storm, etc. This will need to be monitored after storm landfall and carefully measured to determine effects.
That being said; I received a good bit of information from an environmental engineer after publishing the first article and I’m far less concerned about the long-term health effects of any oil dropped on the coast for agriculture, humans, and all other flora and fauna. The oil we’re seeing from the current spill (and especially what remains on the surface after all of the dispersants) is a very light crude and is rather easily broken down by natural processes. Any oil dropped on the coast (or further inland) will most likely not have long-term consequences.
For further reference I pulled up a 2005 study on crops in the Niger Delta region in Africa. This area is known for being one of the most fertile on the continent while simultaneously being an area rich with oil exploration and drilling. As such (and partially due to poor drilling practices) the area has been inundated with oil spills, collecting a cumulative 1.8 million unrecovered barrels spilled over the past forty years. While the study noted a demonstrable effect on crops due to contamination they concluded that a 10% increase in oil pollution corresponded to only a 1.3% reduction in crop yield and a 5% reduction in farm income. The amount of oil present there far exceeds what one would expect to rain down across the southern United States even at worst case; I feel comfortable in saying that we shouldn’t see any long-term soil damages due to any raining oil.
Two caveats to that conclusion include the wetlands of Louisiana and potential short term economic damages. Louisiana’s swamps and easy access waterways are prime targets for a storm surge; it is very possible (and I would conclude probable) that we’ll see at least medium term damages to the wetlands and birds along the coastal plain due to oil inundation if Louisiana gets hit with a hurricane of any substance. High winds and wave action would render booms essentially useless. The second caveat concerns raining oil; if sufficient quantities of oil rain down on crops during harvest they could be ruined economically. Crops may be fit for consumption (and therefore not fit into the “lost” category in a study of African agriculture) but a catalyst of either tainted taste or even publicized concerns over the agriculture’s viability may keep a large portion of the crops in these areas off of the market for at least this year, thereby making “lost” crops in an American definition exponentially higher than the cited study; we tend to be a bit more concerned about food quality than the average African nation. How probable is this? It depends on what stage the crops are in, where the storms hit, and how heavy any oil deposits are in those areas. Again, probably not a health hazard, but it could quite possibly have a significant economic impact on the region.
The fishing industries are another story; fishing, shrimping, oystering, etc are most likely dead industries along the coast for the next few years. Fishing along the Mexican coast was disrupted for two years as a result of the Ixtoc spill in 1979; the English coast saw similar experiences in certain species after the Braer spill in 1993. It looks to me like the amount of oil spilled from Deepwater Horizon will outclass both of those greatly; how long those effects last on the fishing industries remain to be seen.
There is hope, however. One thing I’ve gained an appreciation of in this research is the extent to which oil is biodegradable (the environmental engineer I mentioned earlier informed me that 5,000 barrels of oil leach into the Gulf naturally each day from the ocean floor). Oil is a natural product of the earth; while it isn’t the easiest thing for ecosystems to get along with, especially in larger concentrations, Nature does have processes in place to take care of every drop spilled. Ironically while storm systems may negatively affect land based crops the wave and wind disruptions will act as natural dispersants and help cleanse the ocean of oil. The unfortunate reality for those of us who would like to bring home a paycheck every two weeks is that these processes take time; while the environment will recover, long-term economic damage has already been done in a short period of time.
One of the forms the damage to the fishing industry will take is oxygen deprivation of the water; this occurs due to the microbial breakdown of the oil. As these natural garbage cleaners eat the oil they use oxygen; with the sheer amount of oil spilled and therefore biological breakdown occurring we’re seeing oxygen deprivation in deep water at levels that approach starvation for microbes. This creates what is known as a “dead zone” and takes quite a while for the water to re-oxygenate. Because of this it will take a while for the ecosystem to recover fully enough to support commercial harvesting at any level.
It should go without saying but the tourism industry will probably be negatively affected; my best guess is severe downturn until at least a full year after the well is capped and noticeable effects for years after that. If only due to the connotations of beaches which once had oil spilled on them, any business with ties to tourism should take measures to weather the financial storm that lies ahead.
Dispersants
There’s an incredible amount of controversy regarding dispersants; I’ll do my best to accurately represent both sides then communicate what I’ve concluded.
First, the pro side. Dispersants are used in an effort to force the oil floating on the surface to break itself into small droplets and sink (what the wave and wind action of hurricanes naturally does). The theory is two-fold: that the more surface area available to microorganisms for the purpose of biodegradation of the oil will hasten the process, and that oil below the surface of the water will pose less of a threat to birds and wetlands (at the cost of increasing risk to fish and other undersea organisms). There have been two dispersants used on the oil spill thus far: Corexit 9527 and Corexit 9500. 9527 was used first and then discontinued during this spill at the request of the EPA. Although it is still on the EPA’s approved list it is apparently considered too toxic for them to stomach the use of to any large degree (why it remains on the “approved” list is a great question). Corexit 9500 has been used in its place. The manufacturers of Corexit admit that these are toxic, but to what degree is uncertain. The problems lie mainly in the test data; different types of oil (heavy vs. light crude) react differently; different life forms react differently; they also react differently at different life stages; mechanical action due to wind, waves, and storm affect the dispersant’s effectiveness in various ways, etc. When you throw in all of the variables and look at the history of dispersants one begins to see that they haven’t really been that thoroughly tested. Even the EPA’s own list of legally approved dispersants varies widely in the toxicity levels published.
Dispersants were used widely in the Braer spill in the Shetland Islands in 1993; although study has been done on the health of both the local people and wildlife there’s been no appreciable increase in any diseases that can be directly traced to use of the dispersants. As a side note there was an 800% increase in the number of individuals who “scored above the level at which a subject could be considered a case” for psychological treatment; presumably this would be due to the stresses of the incident. Although “few (fish) were either killed by the oil or suffered serious physiological damage” as a result of it, “two year classes of farmed fish were destroyed.” This was not specifically linked to either dispersants or dispersant laced oil, but rather to the oil spill effects in general.
A great deal has been made about Corexit being banned in the UK; the presumption here is that this is an obviously toxic source and we should do what the Brits do. This assumption may or may not be wrong; it is, however, based on faulty logic. The reason that the UK bans Corexit is that it fails the “limpet test,” wherein a solution of dispersant and oil is sprayed on rocks to see if limpets (a type of small mollusk) can still cling to them. Corexit proved too slippery and was therefore banned. Because the majority of England’s coasts are rocky (and almost none in the Gulf of Mexico are) using this as a basis for comparison is incorrect.
Now for the con side. One of the main ingredients in Corexit is 2-butoxyethanol which, according to its Material Safety Data Sheet, has the following characteristics:
- 2-Butoxy Ethanol can affect you by ingestion and may be absorbed through the skin.
- 2-Butoxy Ethanol should be handled as a CARCINOGEN--WITH EXTREME CAUTION.
- Contact can irritate the skin and eyes with possible eye damage.
- Inhaling 2-Butoxy Ethanol can irritate the nose and throat.
- 2-Butoxy Ethanol can cause nausea, vomiting, diarrhea and abdominal pain.
- Exposure can cause headache, dizziness, lightheadedness, and passing out.
- 2-Butoxy Ethanol may damage the liver and kidneys.
One thing that tends to be very well understood on both sides of the argument is that as toxic to the environment as both oil and dispersants are they are exponentially more toxic when combined into dispersed oil. In fact Dr. Richard Denison, a senior scientist at the Environmental Defense Fund, reveals that independent testing shows that oil that combines with Corexit is four times as toxic as oil and ten times as toxic as Corexit alone. One has to wonder why we’re continuing to use this when independent testing has never shown that dispersant laced oil is safer; the benefit merely lies in its disappearance from view and its supposed speed of breakdown.
According to a 2005 National Research Council study titled Oil Spill Dispersants: Efficacy and Effects, the toxicity of Corexit varies widely among species and life stages. Toxic levels for oyster and fish larvae have been observed as low as 3 parts per million (ppm) for dispersant alone and 1 ppm for dispersed oil (the lower the number the more deadly the chemical). Average toxicity level for approved dispersants trend between 190-500 ppm for dispersants alone and typically 20-50 ppm for dispersed oil. This is frightening when NOAA measurements confirm levels of 100 ppm of dispersed oil in the first half meter of water and 12.5 ppm at ten meters; within which all of the sensitive life stages of sea life are contained according to toxicologist Carys Mitchelmore of the University of Maryland's Chesapeake Biological Laboratory.
Three final concerns: first, that Corexit evaporates readily and could therefore accumulate in rain. There’s no study that I’m aware of that has investigated this, so I couldn’t inform you accurately one way or the other. The second concern is that dispersants could bio-accumulate; this is addressed to some degree in the EPA’s MSDS’ for Corexit: “Based on a review of the individual components, utilizing U.S. EPA models, this material is not expected to bio-accumulate.” Unfortunately they’re judging simply by ingredients and not the compound (which has different properties) and they presume this to be correct. The final concern is listed in the MSDS (published by BP, referenced above) in Section 6 under “Environmental Precautions.” The statement that appears is: “Do not contaminate surface water.” I’m curious as to why more than 1 million gallons of this has been used on surface water alone (as of 1 July) if this is the case.
In the end no one really knows what effect Corexit & Corexit-laced oil will have. There is no doubt it is a toxic substance but the product has been tested to such a small degree while not controlling for even all of the known variables that it’s a crapshoot at best. By the numbers, it looks like Gulf genocide. When one steps back and considers how resilient nature is and how large the Gulf is by comparison the numbers begin to pale a bit though. Even Dr. Dennison’s worst estimates put the current amount of Corexit laid down at killing half of the fish in a 4 square mile area down to the bottom of the ocean, or if diluted sufficiently killing 5% of fish across 40 square miles. In an area as large as the Gulf of Mexico this is trivial. Hopefully these are the only side effects we’ll see.
Gas
Methane is one of the stronger greenhouse gases (21 times more potent than carbon dioxide according to the EPA). With estimates of 4.5-9.0 billion cubic feet leaked from the spill thus far one may begin to wonder about the effect this methane will have on the atmosphere. I checked to see how much methane the U.S. leaks into the air each year; the number projected for 2010 is 186.0 million metric tons of carbon equivalents (MMTCE). At a density of 0.717 kg/m3 at STP the amount of methane gas leaked from the spill is estimated at between 91,000 and 182,000 MMTCE thus far, or between 0.05-0.1% of just the U.S.’s annual contribution, a rather negligible amount.
There have also been concerns that dissolved methane will eliminate oxygen in the waters they’re deposited into; this would create dead zones and negatively impact the surrounding ecosystems for decades. Thankfully it takes about 50 years for methane to oxidize into H2O and CO2; therefore the vast majority of the gas will surface and enter the atmosphere. Dead zones from methane gas in the ocean are not a major concern.
I was mistaken in my first article when I referred to a “methane bubble.” Based upon what I had read at the time I assumed that there was an actual bubble of methane in gaseous form beneath the ocean floor. After interviewing a chemical engineer and subsequent research I found out that the gas is actually in the form of methane hydrate; sometimes called “fire ice” (due to its unique properties of being able to burn while still frozen). This is methane trapped within a cage-like structure of water molecules frozen into a solid chunk of ice; it’s generally a very stable structure as long as temperature and pressure is held constant; usually a reliable notion since methane hydrate most commonly occurs in either permafrost or underneath the ocean floor at depths of greater than 500 meters. Deposits can slowly leak over time but rarely are they disturbed by nature to a sufficient degree to cause readily observable or significant disruptions.
One of the concerns I mentioned earlier was a tsunami triggered by the leak. This is still a concern, but not through the vector I previously wrote about. Since there is no methane “bubble” there is no danger of an eruption from that, and after speaking with a marine scientist I was assured that there is insufficient oxygen at those levels to result in an explosion of any sorts. This is great news, because the sudden expansion of a deposit as large as the methane hydrate looks to be would have triggered a tsunami that probably would have reached dozens to hundreds of miles inland along the coastline; this, however, appears to be quite the impossibility.
I did mention that tsunamis are still a concern. Methane hydrate, as mentioned earlier, is only stable under constant pressures and temperatures; the conditions under which it forms. When either of these changes (generally through a decrease in pressure or an increase in temperature) the hydrate goes through a phase change and becomes a gas. The following quotation comes from the German Advisory Council on Global Change’s Special Report in 2006: “If the methane hydrate stability zone is reduced, then methane gas forms below the hydrate layer. This gas can either penetrate through the hydrate layer and escape out of the sea floor through small channels or permeable sediment layers, or it can blast through the hydrate layer if sufficient quantities of gas collect below a continuously thinning layer. In such a blowout large amounts of methane gas are abruptly released.” Methane hydrate yields 160 times its volume in gas, so you get quite a bit of pressure release; or what we’re seeing with the Deepwater Horizon drill site now with 40-70% of the leak being comprised of methane gas. Every time a cap is attempted the intense pressures from the gas jet out from around the wellhead causing further erosion of the wellhead foundation. What we’re seeing is a rather intense phase change over an obviously significant deposit of hydrate; this is caused by one of two things. The oil coming up is heated by subsurface magma in the earth’s mantle and this temperature change would be sufficient to induce a phase change; the other possibility is that the opening provided by the drilling allowed the pressure of the hydrate deposit to vent sufficiently to allow the phase change. Whichever it is (or a combination of both) we’re seeing a steady erosion of the solids composing the deposit (and thereby the support for the ocean floor above it).
The following flow chart was pulled from the same report. Check out the stimulus on the left side: “Dissolution of hydrates from the bottom upward” which is what we’re seeing now due to pressure and/or temperature change. This of course leads to methane escaping by blowout and could possibly trigger submarine landslides.
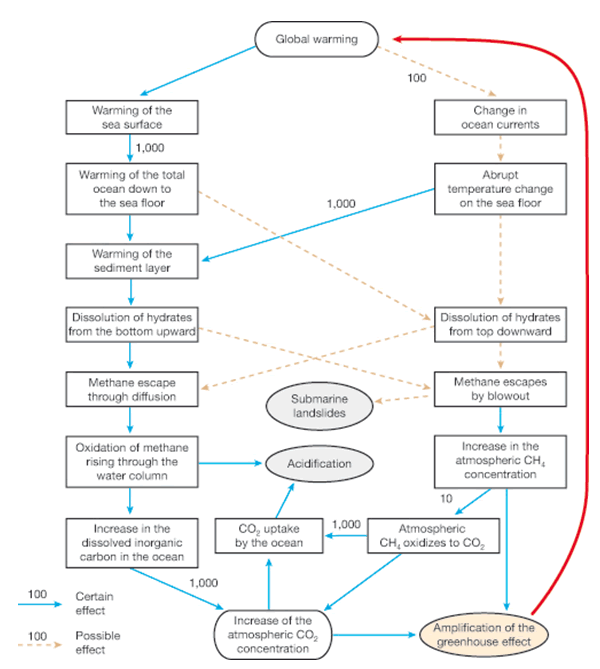
Such a catalyst was eerily predicted by the report four years ago when this was published: “There is already a need today for institutional action with regard to marine methane hydrate deposits. This is with respect to, for one, the targeted mining of marine methane hydrates, and for another to the unintentional release of methane that could occur during sea-floor mining.
Theoretically, efforts to recover methane from hydrates could unintentionally trigger their release into the environment, in the worst case as a sudden eruption. The risks of this have not yet been sufficiently investigated (Archer, 2005). A leak of methane into the environment during mining would unnecessarily amplify global warming. In the worst case even a slope slide could be caused that could trigger a tsunami.”
Because of what the catalyst for the tsunami would be it would most likely head in a different direction than I indicated in my first article. The premise for the tsunami threat I previously wrote about was an eruption or explosion which would trigger wave action in a 360 degree circle; an underwater mudslide would head downhill and project the vast majority of its kinetic energy in that direction.
The oil spill is located on a steep canyon facing south. Any mudslide would therefore push a tsunami primarily south, away from the United States. Whether this is good or bad news depends on where you are as you’re reading this. There would probably be some larger-than-normal wave action generated north of any landslide event; this would be produced by back fill from the depression of the waves and would most likely not be large enough to be called a tsunami north of the slide. Any coastline protected by barrier islands would be that much better off as well.
The German report describes how this would occur: “Methane eruptions can…destabilize continental slopes and trigger large submarine landslides, which can then possibly result in the break-up of additional hydrates. Evidence of such slides can be found on the sea floor. For example, in the Storegga landslide off the coast of Norway around 8000 years ago, an average of 250 m of the continental slope with a width of 100 km were transported downslope (Archer, 2005). This event triggered a tsunami that was at least 25 m high off the Shetland Islands and at least 5 m high along the British coast (Smith et al., 2004).”
I’ve been asked several dozen times why the U.S. and BP didn’t do more on the front end to study the surrounding geology and determine necessary precautions. In yet another haunting quote the Germans proved to be more right than they knew at the time: “The International Seabed Authority, an institution of the international United Nations Convention on the Law of the Sea (UNCLOS), is responsible for methane hydrate deposits as well as for other resources on the sea floor outside the exclusive economic zone. The Authority grants mining licenses and monitors mining operations. Its regulations adopted in 2000 for the exploration of deep-sea mineral resources contain various environmental aspects. This is a starting point for agreement on concrete standards for mining marine methane hydrate on the high seas. In the opinion of WBGU it is furthermore necessary to improve and expand the monitoring system. It is, however, important to note here that so far ‘only’ about 150 countries have ratified UNCLOS, and of those only about 120 countries have ratified the rules governing seabed resources (those who have not signed include, for example, Iran and the USA).”
Conclusion
I want to thank everyone who wrote in and contributed to my requests for information in the first article; even though I think the probability of some of the results outlined therein is far less than when I originally posted it, the threats are still viable (although several appear in different forms). That being said there are some rather severe long-term economic implications across all of the Gulf States. Fishing and tourism industries should expect a severe decline for years; recovery will have to be built from the ground up over the course of what looks like the next several decades before pre-spill strength is achieved. Because of the fact that these are driving industries across Texas, Louisiana, Mississippi, Alabama, and Florida, the rest of the industries in these states should expect to see an economic downturn ripple effect. The impacts will of course be lessened but they will be no less significant. There is a possibility (though how probable remains to be seen) that certain crops could be damaged by any oil-infused rain; even if they remain effectively safe for human consumption they may not remain so legally (it is possible the FDA will forbid any crops from “contaminated” areas to be sold); farmers should be prepared for the possibility that this negative impact may occur. Consumers should be prepared for across the board food price increases this summer; this will especially be true the longer the Mississippi River is impacted as a shipping lane.
The concern still exists that capping the well may be a long way off. The problem BP is facing is not that the pressures in the well are so high that it is impossible to be capped (as I originally thought) but rather that the casing is most likely broken. This causes oil and gas to vector out around the well casing whenever it is shut off, further damaging the wellhead’s attachments to the sea floor. According to Matthew Simmons, energy advisor to George W. Bush and founder of the Ocean Energy Institute an intact casing is necessary for the relief wells to work. If the casing is busted what we’re looking at is trying to seal an increasingly porous and leaking ocean floor, not a wellhead. This makes the case fundamentally different from and incomparable to cases such as the Ixtoc oil well (in addition to being in 5,000 feet of water as verses 160 feet with the Ixtoc). If such indeed proves to be the case more drastic measures may be necessary, such as attempting to collapse the floor around the leak using explosives. This brings up an entire host of issues and I sincerely hope we won’t have to cross that bridge.
By Joshua S. Burnett
Email Josh at jburnett85@gmail.com
© 2010 Copyright Joshua S. Burnett - All Rights Reserved
Disclaimer: The above is a matter of opinion provided for general information purposes only and is not intended as investment advice. Information and analysis above are derived from sources and utilising methods believed to be reliable, but we cannot accept responsibility for any losses you may incur as a result of this analysis. Individuals should consult with their personal financial advisors.
© 2005-2022 http://www.MarketOracle.co.uk - The Market Oracle is a FREE Daily Financial Markets Analysis & Forecasting online publication.


